What is HAMA?
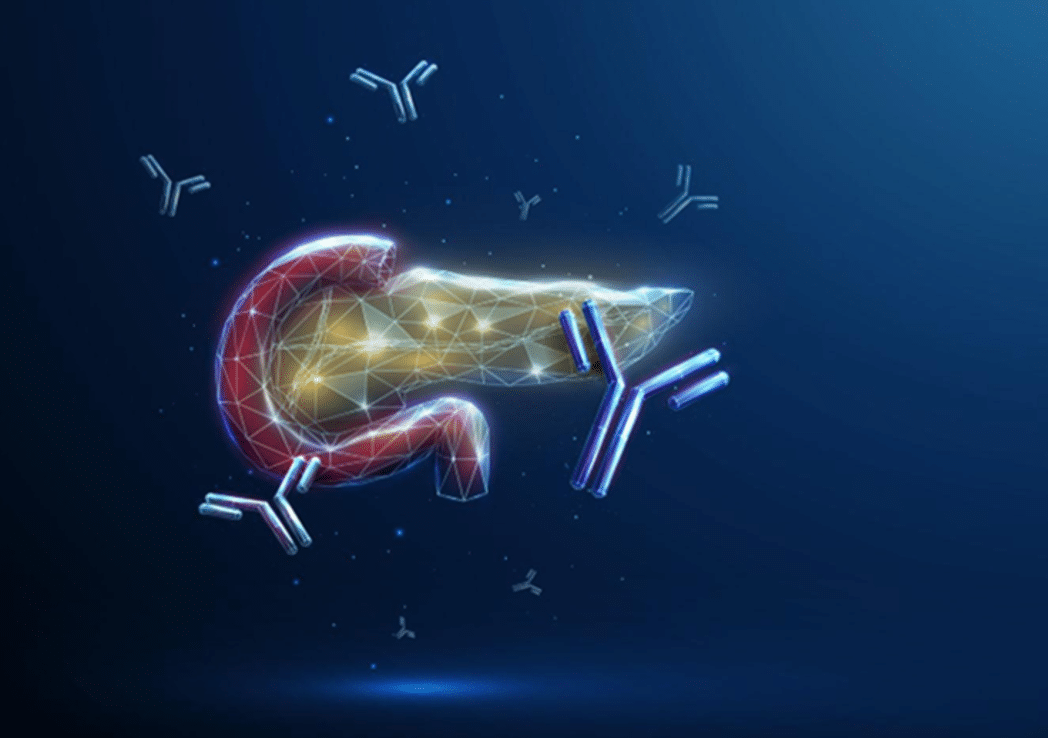
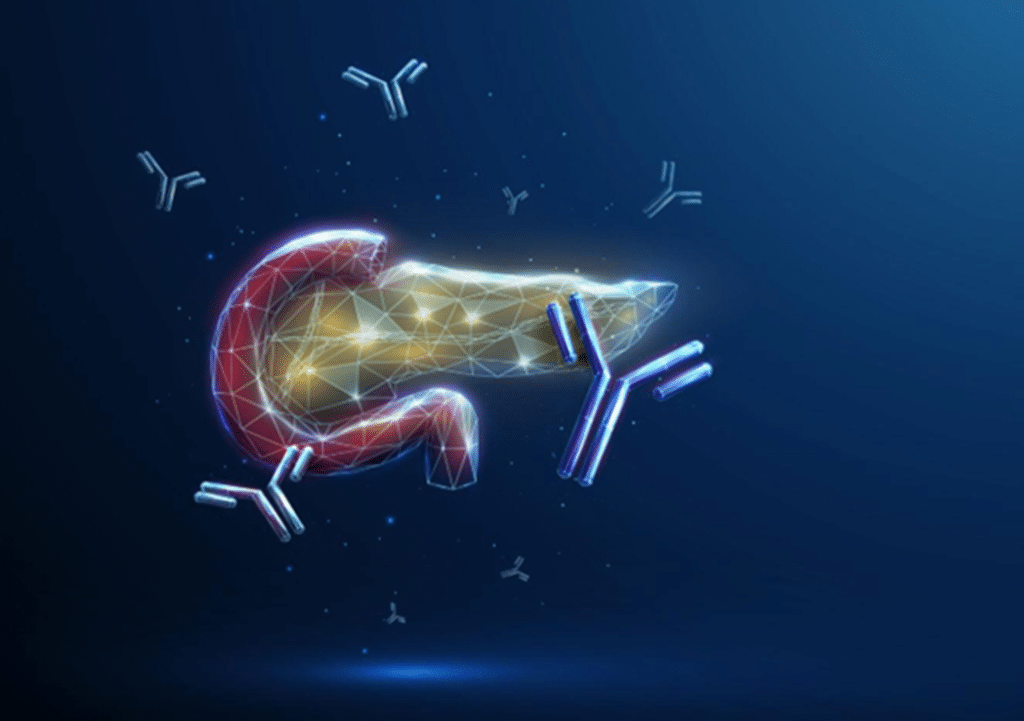
HAMA is an acronym for Human Anti-Mouse Antibodies. Some humans produce HAMA and have it present in their blood. Unfortunately for them, and the in vitro diagnostics industry, the presence of Human anti-mouse Antibodies (HAMA) in patient samples can lead to false positive and false negative results in immunoassays.

Murine MC HAMA
Why do some Humans produce HAMA?
Some people work directly with mice while others might inadvertently encounter mouse proteins or immunoglobulins by coming into contact with mouse urine or contaminated food. These people could develop an immune response against mouse immunoglobulins (antibodies) they encounter such that their immune system produces HAMA.
In the past, mouse monoclonal antibodies were used as therapeutics and could elicit an immune response resulting in the presence of HAMA in human individuals. However, these days monoclonal antibody-based pharmaceuticals are “humanised” to avoid this problem.
Presence of HAMA in individuals is rare, but still needs to be accounted for in immunoassay design.

How can HAMA impact Immunoassays?
When a patient is tested for a condition, the test performed is commonly an immunoassay and the sample that is tested is serum or plasma derived from the patient’s blood. Immunoassays are typically developed using a “matched pair” of mouse monoclonal antibodies to bind to and detect the marker of interest. The ‘marker’ of interest depends on the condition being diagnosed but could be, for example, Troponin I which is a marker of acute myocardial infarction, or HIV p24 antigen which is a marker for the presence of HIV virus. The patient sample (e.g. serum or plasma) is applied to the immunoassay and if the marker being tested for is present the mouse monoclonal antibodies within the assay bind to it and a signal is generated.
However, where HAMA is present in the patient sample the HAMA can bind to the mouse monoclonal antibodies used as immunoassay components and can either i) block the mouse monoclonal antibodies from binding to the marker of interest resulting in a false negative result, or ii) form a bridge between the pair of mouse monoclonal antibodies, generating false positive signal. Where a patient receives an incorrect diagnosis due to the presence of HAMA, the consequences can be devastating. At least 34 cases of hCG false-positive tests in the United States between 1999-2004 resulted in the patients receiving chemotherapy or surgery, including 10 hysterectomies, for assumed cancer1.
How can HAMA interference be prevented in immunoassays?
Assay manufacturers can develop their assays in such a way as to minimise the interference from HAMA, for example by adding excess mouse immunoglobulin to their assay buffers. When this is done, the HAMA present in the patient sample can bind the excess mouse immunoglobulin rather than the reagents being used in the assay.
Immunoassay developers will need to access patient material in order to design an assay that is not affected by HAMA, and also to show that the assay works in the presence of HAMA. Logical Biological is able to provide HAMA-positive serum and plasma.
Even if blockers are used to control HAMA, the heterogeneous nature of its presence in patients mean that it is difficult to rule out its influence entirely, unless it is measured. If HAMA is suspected, the clinical laboratory can perform serial dilutions with an appropriate buffer to demonstrate nonparallelism (higher recovery of the signal than expected).
An alternative solution to controlling for HAMA interference is to use monoclonal antibodies from an alternative species in the immunoassay, in place of mouse monoclonal antibodies. Rabbit and Sheep monoclonal antibodies are candidates here. However, this is not a great solution as some individuals in the population will be producing human anti-rabbit and anti-sheep antibodies. Synthetic peptide binders and/or recombinant antibodies can theoretically avoid the problem of HAMA when used as alternatives to antibodies raised in animals.
Reference
- Human Chorionic Gonadotropin (hCG), By Laurence A. Cole, Stephen A. Butler. Elsevier. 2010.
Want to hear more from Logical Biological?
Sign up to our newsletter to for the latest updates.
Subscribe Now



)